Going natural
Using heart patients' own tissue to repair damaged valves
Nathan Healey was in the prime of his life, a successful tennis pro who had been a contender at the Australian Open, when his heart erupted. A seemingly healthy 32-year-old, he was puttering around his house in Reading, Pennsylvania, keeping an eye on his newborn baby, when he felt a tightness in his chest.
“All of a sudden, I felt dizzy and my heart rate was rising. I guess that is when something blew inside,” Healey, now 37, recalled recently from his home near Sydney, Australia. “I sat down, calmed myself and called my wife. I said, ‘I think I had a heart attack.’”
An ambulance ferried him to the local emergency room, where doctors found that a hole had ruptured in the center of his heart, releasing a stream of blood into his system. Healey was transferred to the University of Pennsylvania medical center, where the cardiac surgeon on call, Joseph Woo, MD, greeted him before midnight with some grim news.
“I remember hearing Dr. Woo say, ‘Chances aren’t good, but I will see what I can do,’” Healey recalls.
As Woo had learned, Healey was born with some previously undetected heart defects, including a weak spot that had progressively enlarged and finally burst open. He had other abnormalities in his aorta, including an aortic valve with three flaps of markedly different sizes, making it hard for the valve to close properly.
In the operating room, Woo faced an urgent decision: Should he try to repair the very malformed and defective valve using Healey’s own tissue, or should he replace it with a mechanical or animal valve, as was the standard procedure? Woo knew that Healey’s athletic career would be over if he replaced the faulty valve, as the replacement options would not be durable enough or would require him to take lifelong medications that would limit his physical activities.
He decided to take the extraordinary step of repairing the valve, doing some creative sculpting. He remodeled the three oddly shaped flaps and used that tissue to fashion two flaps of equal size. He also rebuilt some of the surrounding tissues in a seven-hour procedure that saved Healey’s life and livelihood.
“It was an epiphany,” Woo, who in 2014 became the chair of cardiothoracic surgery at Stanford, says of the experience with Healey, which he later described in a 2013 case report in the Annals of Thoracic Surgery. “We’re always thinking, ‘How do you use what’s there and take advantage of it? That’s the fundamental concept to natural valve repair — to use what’s there in whatever creative manner you can to design something that works.”
As with other surgical specialties, Woo says the growing trend among heart surgeons is to try to recycle, reuse, rebuild and preserve as much of a patient’s own tissues as possible, as patients generally do better when they don’t have to rely on synthetic or animal parts.
“For each of the heart’s components, we and others are developing ways to preserve the tissues and rebuild and resculpt them to take them from a diseased state and return them to a healthy state without removing or discarding things and needing to use artificial or animal substitutes. Your own tissues are alive and can heal and grow,” he says. “That is where we are headed.
“Mr. Healey’s situation is an extreme example where we really pushed this idea to the absolute limits and it was successful.”
That philosophy has put Woo in the forefront of the movement toward natural valve repair, which continues to evolve as surgeons devise new techniques and gain experience. Some in the cardiac community have been slow to embrace the idea, particularly when it comes to the aortic valve, which presents some challenges.
“Aortic valve repair is more complex than replacing a valve. It takes a longer time. It takes more skill,” says Arnar Geirsson, MD, associate professor and chief of cardiac surgery at Yale University. “I think in general cardiac surgeons probably do not repair enough of the valves that could be repaired. But I think eventually there will be more and more centers that specialize in these techniques, and eventually they will become more common.”
While aortic repairs are now being done only in a small number of medical centers with expertise and experience, some see them as the future of cardiac care.
“Aortic repairs are not as standardized and not as frequently performed, and they’re not as successful as mitral valve repairs, but there is progress being made now,” says Bruce Reitz, MD, professor emeritus and former chair of cardiothoracic surgery at Stanford. “I think that’s definitely the way the field is going.”
Valve basics
The heart pumps blood in only one direction, with the heart valves helping to maintain the flow as part of a well-coordinated choreography. With each heartbeat, the valves open and close their flaps, known as cusps or leaflets, first releasing the blood and then closing their doors to prevent it from flowing backward.
The process is regulated by the four heart valves — the aortic, mitral, tricuspid and pulmonary — which open and close as many as 100 times a minute. While all the valves can become diseased, the mitral and aortic valves are most prone to problems, as they control blood flow on the left side of the heart, which carries the heaviest load.
The mitral valve helps move blood from the heart’s upper to lower left chamber and over time can become damaged because of intrinsic genetic abnormalities or through wear and tear. The leaflets may turn floppy and lose their ability to close properly, a condition known as mitral valve prolapse. When a prolapsed valve allows blood to flow backward, surgical intervention may be needed. The valve also can be damaged by a buildup of calcium or plaque, which narrows the space and restricts blood flow, putting excessive demands on the heart.
The aortic valve is the final gateway for blood as it leaves the heart and enters the bloodstream. It is also prone to prolapse and leakage as well as narrowing from calcium buildup, which can thicken and harden the tissue and limit blood flow. This latter condition, known as aortic stenosis, is most common in older adults.
Rheumatic heart disease used to be a common cause of valve problems, and, while still prevalent in developing countries, it has all but disappeared in the United States. Intravenous drug abuse, spurred by the opioid crisis, has become a more common source of valve infections and a growing problem in this country, says cardiac surgeon Duke Cameron, MD, at Massachusetts General Hospital in Boston. Finally, valve problems can result from structural birth defects — the source of Nathan Healey’s crisis.
In the early days of valve treatment, clinicians tried to repair the damaged structure, but had little success, in part because of technological limitations, including the lack of imaging tools to gauge valve leakage, Cameron says. The 1960s then saw the introduction of mechanical valves that enabled doctors to cut out the diseased tissue and replace it with a substitute made of mechanical parts, similar to the valves found in car engines, Woo says. These early versions consisted of a grape-sized ball of plastic inside a metal cage that opened and closed to allow the blood to flow through. These prototypes were improved over time, with more sophisticated versions now made of biocompatible metals such as carbon or titanium.
Mechanical valves are effective and can last a lifetime, but they have a major drawback in that blood tends to stick to them and form clots. Patients with mechanical valves have to take blood-thinning medications, which require regular monitoring to avoid excessive bleeding and stroke.
In the 1970s, another alternative came to the fore: valves taken from pigs or cows. These can work well, but aren’t as durable, particularly when used in younger patients who have greater heart demands. Animal valves can wear out in 10 to 15 years, so patients have to undergo a second replacement and endure the risks of another surgery. Animal valves also carry a small risk of infection, which happens in about 1 percent of cases a year, Reitz says.
Because none of these replacement options is ideal, surgeons have turned to creatively restructuring damaged valves, using the patients’ own tissue.
“The idea of valve repair has come back because we’ve lived with these artificial valves for several decades and are beginning to appreciate their limitations,” Cameron says. “Most people don’t want to return for more surgery, and they don’t want to take blood thinners. The gauntlet’s been thrown down to do a better job of repairing valves so patients can have a good quality of life and have fewer reoperations.”
‘The gauntlet’s been thrown down to do a better job of repairing valves so patients can have a good quality of life and have fewer reoperations.’
In 1983, noted French surgeon Alain Carpentier, MD, PhD, published a landmark paper in the Journal of Thoracic and Cardiovascular Surgery in which he described his repair technique, known as the French correction, for mitral valves. He advocated cutting away portions of the diseased structure; shortening the cordlike tendons that help support it and connect it to the left ventricle; then stitching a metal and fabric ring around the base to give it stability.
His work was followed by other developments in the field, including the use of Gore-Tex to create new cords to suspend the valve and to rebuild some of the supporting structure. These innovations led to a surge in the popularity of mitral repair. A 2009 study of some 58,000 patients found the number of repairs done between 2000 and 2008 rose from 51 percent to 69 percent. At the same time, use of mechanical valves fell from 68 percent to 37 percent, according to the report, published by the Society of Thoracic Surgeons.
Multiple studies have shown that patients who undergo mitral valve repair do better overall: They are more likely to survive, spend less time in the hospital and suffer fewer complications, such as infection and stroke, compared with those with substitute valves, whether animal or mechanical.
“With the mitral valve, the data is quite clear. If you can repair, it’s better. You have better survival,” says Geirsson at Yale. “I think once the techniques are improved, that is what is going to happen with the aortic valve.”
Fixing the aortic valve
The guidelines of the American College of Cardiology and the American Heart Association recommend repair for certain mitral valve problems, such as a prolapsed valve, but for other conditions it leaves the decision largely to the surgeon’s discretion, says T. Sloane Guy, MD, associate professor of cardiothoracic surgery at Weill Cornell Medicine. For aortic valve repair, the guidelines are more vague, indicating repair can be considered in “appropriate patients, where good results are expected,” Guy says.
That’s because the aortic valve is a totally different animal, both in form and function. For instance, while the mitral valve has two leaflets, the aortic valve has three, so a surgeon has to effectively line up three sides for the valve to work well, Woo says. There is also less tissue to work with in an aortic valve repair, and different techniques and finer sutures are needed, he says.
With these challenges and a steep learning curve, aortic valve repair has been slower to catch on.
“It’s not been nearly as successful or widely adopted because it’s really a fundamentally different kind of valve, and the results haven’t been as good as mitral valve repair. It really depends on the nature of the particular valve and the particular patient,” says Guy, who encourages patients to go to a center that focuses on repair and has a high volume of cases.
In 2016, Cameron penned a commentary in the European Journal of Cardio-Thoracic Surgery in which he lamented the elusive nature of aortic valve repair, calling it a “difficult nut to crack.”
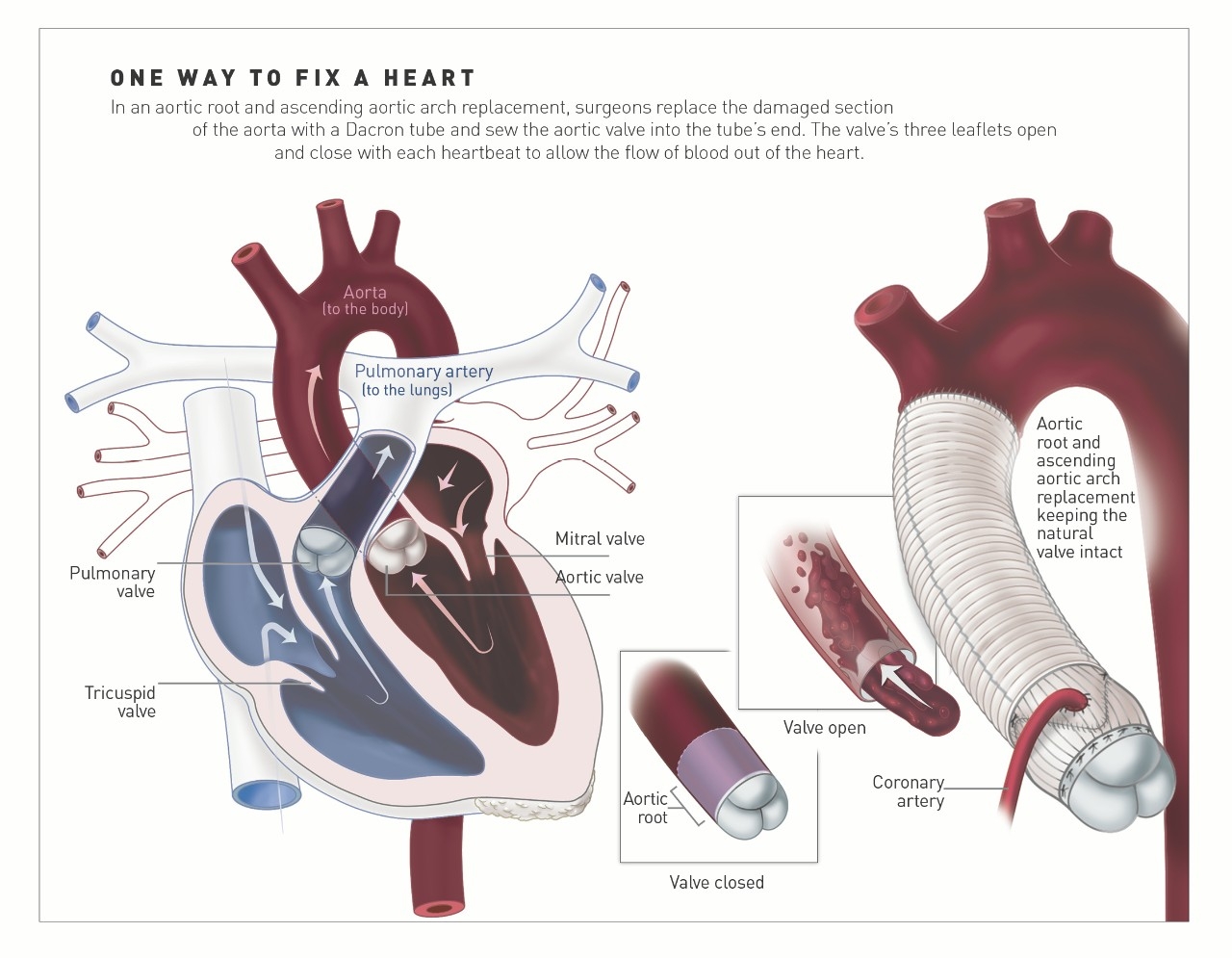
“But there is renewed interest and considerable progress on this front,” he notes, spurred in part by the pioneering work of two surgeons, Tirone David, MD, and Sir Magdi Yacoub, MD. In the 1990s, they developed procedures to preserve the valve in patients with aortic aneurysms, or a bulge in the aorta that can cause it to rupture. The procedure, known as valve-sparing root replacement, is most commonly done in patients with Marfan’s syndrome, a connective tissue disease that can impact the heart, as well as patients with high blood pressure that has led to enlargement of the base of the aorta, known as the root.
In the procedure, surgeons cut out the diseased part of the aorta and replace it with a tube of Dacron polyester, known as a graft, which is stitched to the heart. Instead of cutting out the aortic valve, as was done in the past, surgeons preserve the patients’ tissue and reimplant it inside the new tube, sometimes refashioning the valve to fit the space. The procedure is made more complicated by the fact that the surgeons have to detach the coronary arteries, which flow from the aorta, and then reattach them once the new graft is in place.
In the operating room
On a recent September morning, Woo is called in to perform a variation on this procedure at Stanford Hospital in a man in his 50s who has endocarditis, a heart infection, which has damaged his aortic valve and aorta. The patient is put on a heart-lung bypass machine, which temporarily stops his heart and takes over the function of his heart and lungs while the surgical team does its work. Before Woo begins, he views the heart on an echocardiogram, displayed on a nearby screen. It shows the valve leaflets flopping back and forth. “Wow, there’s significant leaflet destruction there,” he says.
He and his team, including David Scoville, MD, chief resident in cardiothoracic surgery, begin by cutting out the defective aortic root, then meticulously excising 20 small fragments of diseased tissue from within and around the faulty valve. They replace the aortic root with an inch-wide Dacron tube, which they anchor in place with multiple blue nylon sutures. Then comes the most challenging part —sewing what remains of the patient’s valve back inside the tube.
“Imagine tailoring a suit but from inside the suit,” says Woo, as he does some customized tailoring inside the narrow tube, stitching the valve in place and shaping it with fine Gore-Tex sutures so the cut leaflets are reassembled and then evenly aligned. The process is painstaking: He carefully loops in the thin threads, using fine tools. It’s a procedure many surgeons won’t attempt, as there is very little valve tissue left to work with. But for the patient’s sake, Woo is determined to make it happen.
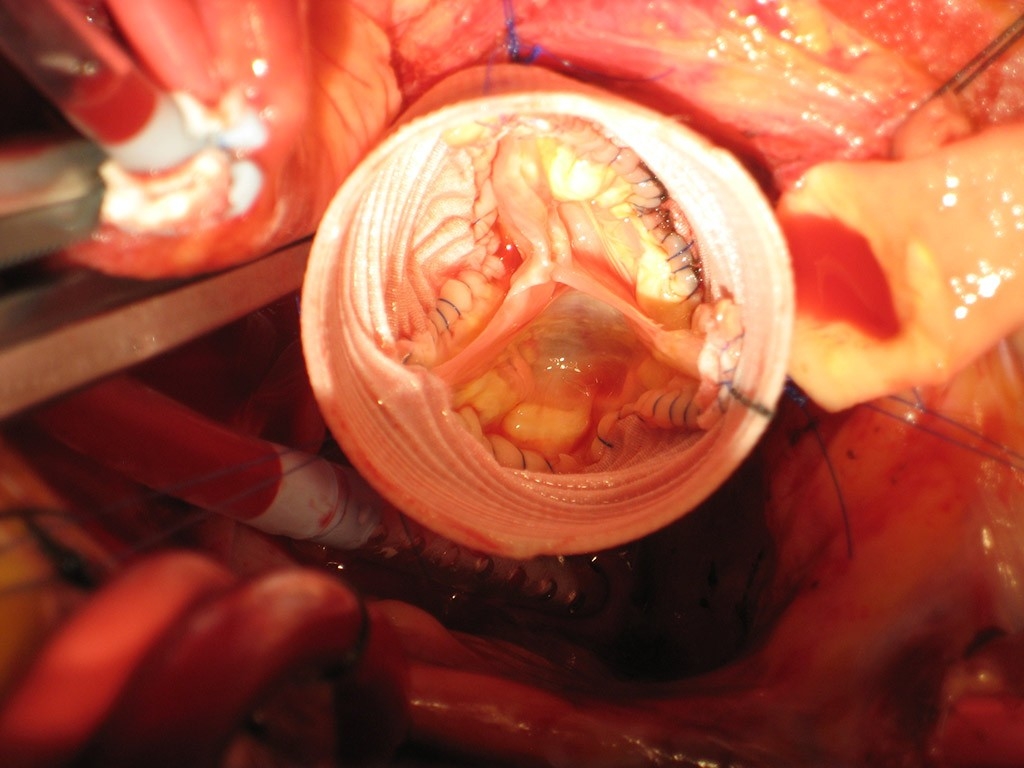
He and his colleagues then create two small holes in the graft to reattach the coronary arteries. They begin with the left main coronary artery, which Woo lightly calls “the seat of the soul,” as it carries blood to nourish the muscles of a vital heart chamber which pumps blood to the entire body. They then reconnect the right coronary artery and pressurize the repaired aortic valve while surveying the results on the imaging screen, which shows a normal valve opening and closing with leaflets neatly lined up.
“It’s opening up nicely and closing beautifully,” Woo says after a process that takes more than four hours. “This patient will keep his own valve over time.”
A few weeks earlier, Woo tackles a different type of repair, fixing a mitral valve in a patient in his 60s who has had a prolapsed mitral valve for decades. Recently, the man began to have problems, including shortness of breath and palpitations, with tests showing the valve is leaking blood. In the operating room, the patient is put on a heart-lung bypass machine, but instead of opening up the chest, Woo is able to use a minimally invasive approach, accessing it from the side through a 2-inch incision between the ribs. This approach is as effective as operating through a major incision in the chest and reduces complications, pain, blood loss, infection and scarring, as well as time spent in the hospital, he and Andrew Goldstone, MD, a cardiac resident, reported in a 2016 review paper published in Annals of Cardiothoracic Surgery.
Woo and his colleagues, including cardiac fellow John MacArthur, MD, operate through this small opening, reviewing their progress on the video screen. The mitral valve comes into view — the leaflets looking like puffs of pale, loose tissue. “A lot of surgeons will cut this out,” Woo says. “We are going to resculpt these leaflets to make them as normal as possible.”
Using a procedure he devised, Woo uses a single Gore-Tex suture to further anchor the valve in place and secure it to the wall of the ventricle. He folds in the excess tissue from the leaflet to prevent it from prolapsing again. He then takes a titanium ring covered in dark fabric and stitches it down around the base of the valve using spaghettilike sutures. The procedure tightens up the valve and eliminates the leakage.
Once done, Woo reviews his handiwork on the imaging screen, confirming that there is no backflow of blood — the valve is effectively repaired. He is intensely focused and works efficiently, so the whole process takes about an hour and a half, making a procedure that can take some surgeons as much as six hours look relatively easy. In a 2013 study in the European Journal of Cardio-Thoracic Surgery, he and MacArthur found that patients undergoing this version of mitral repair spent only 59 minutes on a heart-bypass machine, almost half the usual average, and did very well. This patient of Woo’s recovers nicely and is able to leave the hospital four days later.
In general, surgeons tend to develop their own variations on these repair techniques, depending on the patient’s anatomy and the availability of tissue for resculpting. Unlike other clinicians who are subject to rigid guidelines in prescribing drugs and other treatments, surgeons have a great deal of leeway in adapting their practices based on what they encounter in the operating room.
All-repair philosophy
Woo says he likes to approach each patient as a candidate for repair, though he realizes it’s not always possible. For instance, in patients with valve fibrosis, the leaflets may be so thickened and damaged by calcium deposits that they can’t be manipulated and preserved. But he is nonetheless guided by an all-repair philosophy.
“We believe, in our hands, we can try to approach everyone as potentially reparable,” he says. “No one should automatically be viewed as not being a repair candidate. Everyone should have an opportunity.”
He says he often gives talks to cardiologists and cardiac surgeons throughout the world, trying to promote the concept and techniques of repair. While there is interest and curiosity, he says he also has met with some skepticism, as these surgical techniques are new and can take years to master. Some are simply resistant to change.
“It’s an ongoing challenge to educate the community that aortic valves can be repaired,” Woo says. “Either they have never heard of it or they’ve never seen it done effectively by a surgeon. Or they don’t want to try it out until there is long-term durability data,” which is not yet available.
He is also training the next generation of surgeons — people like Goldstone, now a resident in cardiac surgery at the University of Pennsylvania, who has been working with Woo over the past seven years both at Stanford and Penn learning these approaches.
“We are trying to train him and others at a very young age so they will carry on and further advance these complex reconstructive techniques,” Woo says. “Through those whom you educate and train, you create a pathway for benefiting society for many decades down the road.”
Healing Healey
As for Nathan Healey, he fully recovered from his marathon repair procedure after spending 10 days in the hospital. Woo implanted a pacemaker in his heart, as the rupture disrupted its natural rhythm. Healey was able to return to professional tennis and three years later went on to try his hand in the 2015 U.S. Open. He says he is likely one of the few players with a pacemaker to compete at that level.
In the fall of 2016, he moved with his family back to his native Australia, where he now coaches tennis, starting each session with a meditation. He also teaches yoga, surfs and competes in the occasional tennis tournament. He has the perspective on life that comes from being close to death, he says, trying to maintain a sense of balance and relishing every day.
Occasionally, he says, there is “this little fear that arises,” the panic that his heart could fail again, but he is monitored regularly, and his doctors have assured him it’s not likely to happen.
“I’m just incredibly grateful to be enjoying the life I’m living,” he says. “A lot of fortunate pieces fell into place that night. I was lucky to get the surgeon and I was lucky to get the repair.”