When bones collide
An unexpected fuel for osteoarthritis
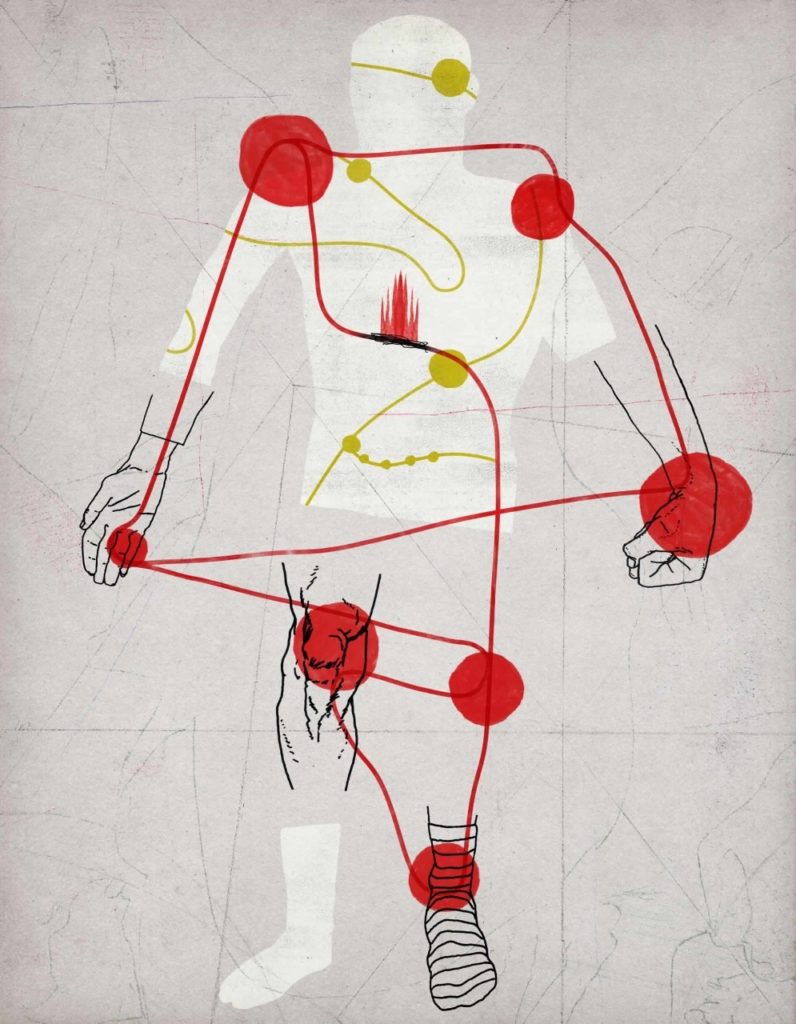
That which does not kill you can still be plenty debilitating. A perfect example is osteoarthritis, essentially the scraping of bones against one another when one of evolution’s carefully crafted masterpieces, a joint, gradually breaks down. An estimated 27 million people in the United States are diagnosed with osteoarthritis, by far the most common form of arthritis. That number is likely to increase to 50 million by 2030, due mostly to the aging of the population.
Over half of the U.S. population has symptomatic evidence of osteoarthritis by age 65, says Bill Robinson, MD, PhD, an associate professor of immunology and rheumatology at Stanford. “Virtually everybody gets it, if they live long enough,” he says. “Some of us get it while we’re still young, typically stemming from an injury to the affected joint years earlier. But far more of us get it later on.”
Who? Everyday people. “You, me, our next-door neighbor,” says Mark Genovese, MD, professor of immunology and rheumatology. Genovese, who sees a lot of osteoarthritis patients in his Stanford practice, describes the condition as ubiquitous. “As we age, it approaches 100 percent prevalence.”
You start to feel some combination of pain, stiffness and tenderness in a thumb, a knee, a hip, a toe or perhaps your back or neck. It takes root, settles in and, probably, gets worse. And once you’ve got it, it never goes away. Eventually, it can get tough to twist off a bottle cap or to get around, depending on the joint or joints affected.
Anything so common is all too easy to look at as simply another of life’s inevitabilities, says Robinson. “People in the field tend to think of osteoarthritis as a matter of age-related wear and tear, like the tires on your car gradually losing their treads.”
But Robinson, who also spends time seeing osteoarthritis patients, has another, revolutionary thought: that far from being the passive product of a lifetime of joint usage, the disease is driven by chronic, low-grade inflammation. He and his colleagues have shown in laboratory studies that components of the immune system are hyperactive in osteoarthritic joints and that stopping low-grade inflammation in its tracks can substantially counter disease progression.
This new thinking about osteoarthritis as what he calls an auto-inflammatory disease instead of a set of bald tires has the potential to change the picture considerably for patients. Having proved his hypothesis first in vitro at the laboratory bench and then in animal models that inflammatory processes are key to osteoarthritis progression, Robinson has passed the torch to Genovese: a classic translational-medicine handoff, from basic research through animal models to clinical trials involving osteoarthritis patients.
That which does not kill me gets neglected
In a sense, osteoarthritis is to movement as Alzheimer’s disease is to memory.
“We can’t stop it. We can’t cure it,” says Genovese, who is also the James W. Raitt Professor of Medicine. “So instead we try to reduce pain in those who have it. But we shouldn’t mistake that for altering the course of the illness, which we are unable to do. We lack any therapies that fundamentally alter the progression of osteoarthritis.”
Loss of function in an osteoarthritic joint is typically accompanied by pain, particularly at night or with overactivity, that can range from mild to utterly disabling. The only treatments today are painkillers and, ultimately, joint replacement. Osteoarthritis accounts for one out of every four visits to a primary-care physician’s office.
With so many sufferers, you might think osteoarthritis would be a hot research area. And you would be wrong. While osteoarthritis may be a huge public-health problem, it’s not going to kill you today. So, says Robinson, it takes a backseat to cardiovascular disease, cancer, AIDS and other more lethal disorders.
“We believe we can change this paradigm that not much can be done to reverse, halt or even slow the progression of osteoarthritis,” says Genovese, who is leading a pilot study in actual patients to test Robinson’s theory. “The prevailing view today is that this is simply the result of the normal destruction that takes place in the body with aging and injury. We’re saying, yes, but the process perpetuates and feeds on itself. If we can disrupt that, we might be able to change the course of the illness.”
“Low-grade inflammation is more like overheated electrical wiring responsible for a smoldering carpet.”
A key component of any joint is cartilage, a shock-absorbing material that lines and separates the two bones defining a joint so they don’t scrape together when we move. Osteoarthritis involves the breakdown of cartilage, the growth and expansion of the bone facing the joint, and the infiltration of inflammatory white blood cells; it also involves increases in inflammation-associated substances in the synovium, the surrounding pouch that supplies the relatively blood-free cartilage with nutrients. It’s not hard to imagine how either a sudden injury to a joint or the strain of its routine use over the decades, compounded perhaps by carrying extra weight, could produce osteoarthritis. But then again, not all old people — or all obese people, for that matter — get it.
Osteoarthritis is distinct from the far rarer rheumatoid arthritis, which affects no more than 1 in 100 people. The latter is an autoimmune condition that occurs when the body’s immune system mistakes proteins associated with the joints for those of a foreign invader and mounts intermittent, escalating attacks on innocent tissue. Leading the charge are the cellular and molecular warhorses of the so-called adaptive immune system: for example, antibody-secreting B cells and toxin-secreting T cells.
It turns out that rheumatoid arthritis and osteoarthritis are examples of overdrive in two quite different branches of the immune system.
A key feature of the adaptive immune system when it’s working correctly is that it precisely targets specific features of the enemy it seeks to fight, thereby sparing innocent-bystander cells in adjacent and surrounding tissues. The adaptive immune response takes a week or two to get up to speed after it detects the presence of an invading organism or tumor-cell wannabe. That leaves a two-week window during which a pathogen, left unchecked, could multiply in the body to the point where it would be impervious to the adaptive immune system’s eventual response.
Filling the gap is another, far more ancient branch of the immune system. Whereas any creature with a backbone (i.e., vertebrates) has an adaptive immune system, all multicelled creatures, from sponges to space cadets, are endowed with a so-called innate immune system. This ragtag army of cell-surface receptors, circulating warrior proteins and primitive, amoeba-like cellular thugs doesn’t customize its every response to the specific features of each offending invader. Instead, upon finding that an unfriendly bacterium or virus is afoot, it quickly mounts a take-no-prisoners response, which we call inflammation: the “calor,” “dolor,” “rubor” and “tumor” (heat, pain, redness and swelling) recorded by the Roman encyclopedist Celsus in the first century and recalled by any modern who ever skinned a knee as a careless kid or sliced a finger instead of a carrot as a harried adult. That inflammation keeps offenders at bay while the adaptive immune system is taking its time revving up. But it also wreaks collateral damage and draws in additional immune reinforcements, taking a toll on the tissue in the vicinity. Up close, not so pretty.
Rheumatoid arthritis occurs when the adaptive immune system mounts an inappropriate attack on native proteins in joint areas, while osteoarthritis, Robinson says, is an example of the collateral damage wrought by the friendly fire of inflammation. Given the quite different immune entities involved, it’s perhaps not surprising that drugs that are effective for rheumatoid arthritis don’t do much for osteoarthritis, says Robinson, who refers to the latter as an “auto-inflammatory” rather than an autoimmune condition.
Robinson further distinguishes between what he calls high-grade versus low-grade inflammation. “High-grade inflammation — what you see in an autoimmune disease such as rheumatoid arthritis — is analogous to a building that’s on fire, with flames coming out of the windows,” he says. “Low-grade inflammation is more like overheated electrical wiring responsible for a smoldering carpet.” Fluid from a rheumatoid-arthritis patient’s affected joint contains 10 times as many white blood cells and immune-signaling chemicals as fluid from that of an osteoarthritis patient, he says.
Chronic low-grade inflammation is now known or strongly believed to be a driver of several other diseases once not associated with immune causation. Among them are cardiovascular disease, macular degeneration, type-2 diabetes and, lately, Alzheimer’s disease.
Next door to Robinson’s laboratory, in fact, sits the lab of neurology professor Tony Wyss-Coray, PhD. About a decade ago, Wyss-Coray began studies of neurons, laying the groundwork for the now widely accepted notion that chronic low-grade inflammation plays a key role in Alzheimer’s disease. His thinking exerted an important influence on Robinson, though Robinson was primed to find it persuasive. “As an immunologist my first impulse is to see everything as an immunological problem anyway, so it came naturally,” he says.
It’s not that Robinson came to the conclusion that injuries or age-related wear and tear have nothing to do with the development of osteoarthritis. Rather, he thinks that those events trip off what becomes a vicious cycle whereby the resulting low-grade inflammation causes further damage to the injured or worn-down joint, for example by breaking apart cells whose spilled-out chemical contents spell trouble to the indiscriminate, innate immune system. In susceptible individuals, that cycle persists, producing one Pyrrhic victory after another by the innate immune system over a person’s own joint tissue.
In a study published in Nature Medicine in late 2011, Robinson and his team demonstrated compelling evidence that inflammation is hard at work in osteoarthritic joints. First, they compared joint fluid from osteoarthritis patients with joint fluid from people without the condition. They saw numerous differences, including increased amounts of inflammatory proteins and significant upticks in the activity levels of several genes associated with the innate immune system.
In particular, the scientists noticed a general rise in the levels of a number of constituents of a 20-odd-protein complex called the complement system. This key component of the innate immune system is already plenty abundant in healthy people, accounting for about 4 percent of all the proteins in our blood. It consists of numerous closely coordinated proteins that serve as sentinels, snipers, snitches and switches amplifying or damping other players in the overall immune response, whose interactions unfold in a carefully orchestrated sequence called the complement cascade.
Next, Robinson and his associates induced osteoarthritis in experimental mice by injuring a cartilaginous shock absorber in their knees called the meniscus. (We have them, too. People with meniscal tears — about 50 percent of all of us over age 60 — are at heightened risk for osteoarthritis. Athletes and others who have meniscus surgery due to a torn meniscus develop arthritis at a rate five- to tenfold that of people who don’t.)
Sure enough, the mice developed osteoarthritis in their wounded knees. But bioengineered mice deficient in complement-cascade-accelerator proteins were dramatically resistant to osteoarthritis progression, incurring only about half as much knee damage from the meniscal injury as normal mice did. Meanwhile, other bioengineered mice lacking a complement-inhibitor protein, and therefore sporting a souped-up innate immune system, got worse faster than the normal mice.
That shook things up in the research community. “Bill was very persistent,” says Robert Terkeltaub, MD, chief of rheumatology at the Veterans Administration Medical Center in San Diego and professor of medicine and division director at UC-San Diego. “The entrenched dogma was that these proteins couldn’t be important in osteoarthritis because they weren’t abundant enough in joint fluid. He showed otherwise. I have the highest regard for what he did. It was truly paradigm-shifting.”
The discovery, and continuing progress in Robinson’s lab, justifies expanding the research focus from animal models to humans. That’s where Genovese comes in. His pilot study will test whether dialing down the low-grade inflammation in osteoarthritis patients can slow or halt progression of the disease.
Can you teach an old drug new tricks?
Medical science is not in the business of bioengineering people. But drugs can often achieve the same purpose. If you’re going to inhibit the complement system, you have to be careful, because complement is absolutely vital in the first days after an infection. Without it, you’d be toast before your adaptive immune system can fire up.
Various experimental anti-complement drugs have entered clinical trials for other indications, but none has really worked out yet. But in Robinson’s experimentation with animal models, a combination of two drugs with anti-inflammatory properties — atorvastatin and hydroxychloroquine (more typically thought of as treatments for high cholesterol and malaria, respectively) — had beneficial effects. Both drugs were long ago approved by the FDA for other indications, known to be safe enough for long-term use, and are now off patent and, therefore, cheap. Importantly, using the two drugs in combination gave better results than you might predict from simply adding their separate effects.
Genovese’s study — funded by SPARK (Stanford’s in-house bioscience incubator) and the Northern California Arthritis Foundation — has enrolled eight patients to date and is recruiting to expand to 16 patients, all over age 35, otherwise reasonably healthy, and showing some evidence of osteoarthritic knee damage (but not so much that hope of some repair is unreasonable) as well as signs of inflammation in that joint. Trial subjects are receiving daily oral doses of both atorvastatin and hydroxychloroquine for four months and being monitored for an additional two weeks after that for evidence of improvement in joint function, pain and the biochemical and radiological condition of their affected joints. It’s far too early to draw any conclusions because no results have been reported yet. Even with good outcomes, says Robinson, a slam-dunk proof that the drug combination safely counters osteoarthritis progression could still be five to eight years off.
Meanwhile, Robinson’s lab is chasing osteoarthritis’ inflammatory triggers upstream. “Specific molecular events appear to be very important in the inflammatory chain of events that drives osteoarthritis progression,” he says. “We are intent on learning exactly how, and why.”
That would be nice. A 2007 study estimated that annual costs to society then were $180 billion including visits to doctors’ offices, the stopgap medications that at least diminish pain, time lost at work and major operations such as joint replacements (running at $50,000 to $75,000 per procedure). Those costs are surely greater now, and with an aging society, they will keep growing unless medical research generates some way of slowing or, better, reversing osteoarthritis’ advance or preventing it from getting a toehold (or a knee-hold or a hip-hold) to begin with.