Children’s vaccine trials
Response of youngsters is being evaluated
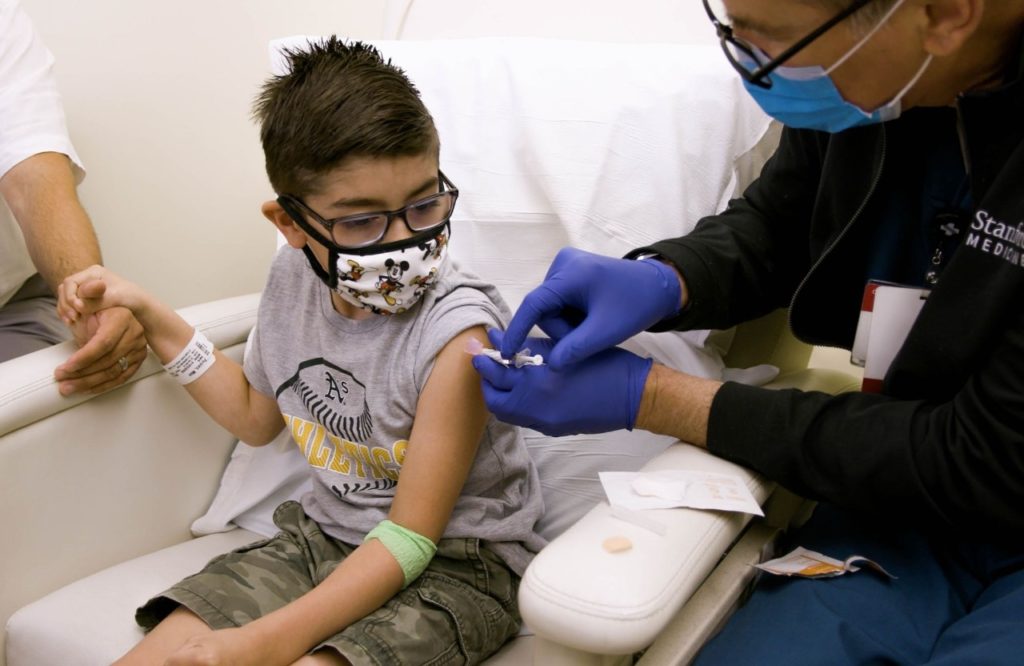
Stanford Medicine is participating in clinical trials to evaluate the response of children under 12 to the Pfizer-BioNTech COVID-19 vaccine.
Since May, researchers have tested whether the vaccine produces an immune response and prevents COVID-19 in children 5 through 11 years old.
The Stanford study site is also evaluating vaccine dosages for children 6 months through 4 years old.
Final results for the older age group are expected later this year.
“Children under 18 make up about a quarter of the U.S. population, so if we want to get the virus under control, we really need to include them,” said Yvonne Maldonado, MD, who is running the trials’ Stanford site.
Maldonado is the Taube Professor in Global Health and Infectious Diseases at Stanford.